[hfe_template id=’470′]
Third Party Opposition against Sanofi’s Patent Application no. BR 11 2017 004197-9 (Sanofi 2)
Sanofi seeks to perpetuate an undue protection in the field of vaccination against Dengue.

Sanofi 2 (Patent Application no. BR 11 2017 004197-9)
Patent Application no. BR 11 2017 004197-9 does not merit being granted, as it is contrary to many of the legal requirements set forth on Public Law no. 9.729/96 (Industrial Property Statute – LPI)
Unpatentability of Therapeutic Methods
Art. 10, VIII, of the LPI

Insufficiency of Disclosure
Art. 24 of the LPI

Lack of Clarity and Support of the Claims
Art. 25 of the LPI

Lack of Novelty
Art. 8º and 11 of the LPI

Lack of an Inventive Step
Art. 8º and 13 of the LPI
Sanofi 2 – Unpatentability of Therapeutic Methods
The current claims of the Sanofi 2 application clearly attempt to protect therapeutic methods – which is in explicit violation of the Industrial Property Statute
-
Industrial Property Statute (LPI)
Article 10 – The following are not considered to be inventions or utility models: […]
VIII – operating or surgical techniques and therapeutic or diagnostic methods, for use on the human or animal body; […] -
BRPTO’s Resolution no. 169/2016
1.27 – Therapeutic methods are those that imply the cure and/or the prevention of a disease or disorder of the human or animal body, or the relief of symptoms of pain, suffering and discomfort, for the purpose of reestablishing or maintaining the normal health conditions thereof.
-
Text of Claim 1
“A vaccine composition for use in a method of protecting a human subject against dengue disease caused by a dengue virus of serotype 2 […]”
-
Text of Claim 12
The composition for use according to any one of claims 1 to 11, characterized in that said method comprises the administration of said composition in three or more doses.
-
Text of Claim 13
The composition for use according to any one of claims 1 to 11, characterized in that said method comprises administrating said composition in two doses, in an interval of three to six months.
Sanofi 2 – Insufficiency of Disclosure
The current claims of the Sanofi 2 application cover subject matter which is not supported by the specification. Thus, the Person of Ordinary Skill in the Art (POSITA) has no means of reproducing the technology without incurring in undue experimentation.
-
Industrial Property Statute (LPI)
Article 24 – The specification must describe the subject matter clearly and sufficiently so as to enable a person skilled in the art to carry it out and to indicate, when applicable, the best mode of execution.
-
BRPTO’s Resolution no. 169/2016
2.13 The descriptive sufficiency must be assessed based on the specification, which must disclose the invention in a sufficiently clear and precise manner so as to be reproduced by a person skilled in the art. The specification must contain enough conditions that ensure the achievement of the claimed invention.
-
BRPTO’s Resolution no. 169/2016
2.15. In this context, it must be ensured that the application contains sufficient technical information to enable a person skilled in the art to:
put the invention as claimed into practice, without undue experimentation, and
understand the contribution of the invention to the state of the art to which it belongs.
By undue experimentation it is understood when a person skilled in the art, from the disclosed in the invention, requires additional experimentation to carry it out.
What is covered by the independent claims of Sanofi 2?
-
Independent Claim no. 1
A vaccine composition for use in a method of protecting a human subject against dengue disease caused by a dengue virus of serotype 2,
said composition characterized by comprising:
(i) a dengue antigen of each one of serotypes 1 to 4, and each one of said dengue antigens of serotypes 1 to 4 are independently selected from the group consisting of:
(a) a live attenuated dengue virus; and
(b) a live attenuated chimeric dengue virus, wherein said live attenuated chimeric dengue virus is a flavivirus receptor in which the nucleic acid sequences have been modified by the change of the nucleic acid sequences encoding prM and E proteins of the flavivirus by the corresponding sequences of a dengue virus;
wherein said method of protecting results in a statistically significant reduction in the incidence or likelihood of dengue disease caused by the dengue virus of serotype 2 and wherein said human subject is immune to dengue.
-
Independent Claim no. 15
Use of
(i) a dengue antigen of each one of the serotypes 1 to 4, and each one of said dengue antigens of serotypes 1 to 4 are independently selected from the group consisting of:
(a) a live attenuated dengue virus; and
(b) a live attenuated chimeric dengue virus, wherein said live attenuated chimeric dengue virus is a flavivirus receptor in which the nucleic acid sequences have been modified by the change of the nucleic acid sequences encoding prM and E proteins of the flavivirus by the corresponding sequences of a dengue virus;
said use characterized by being for manufacturing a vaccine composition and/or a kit for protecting a human subject against dengue disease caused by a dengue virus of serotype 2, wherein said method of protecting results in a statistically significant reduction in the incidence or likelihood of dengue disease caused by the dengue virus of serotype 2 and wherein said human subject is immune to dengue.
-
Independent Claim no. 16
A kit characterized by comprising a composition as defined in any one of claims 1 to 14.
What is actually disclosed in the specification?

Example 1
(Click image to amplify)
This Example only demonstrates the use of a tetravalent CYD vaccine produced and cultured according to a list of prior art documents which exclusively disclose the ChimeriVax concept. Therefore, it does not demonstrate any other “live attenuated dengue viruses” or “live attenuated chimeric viruses”
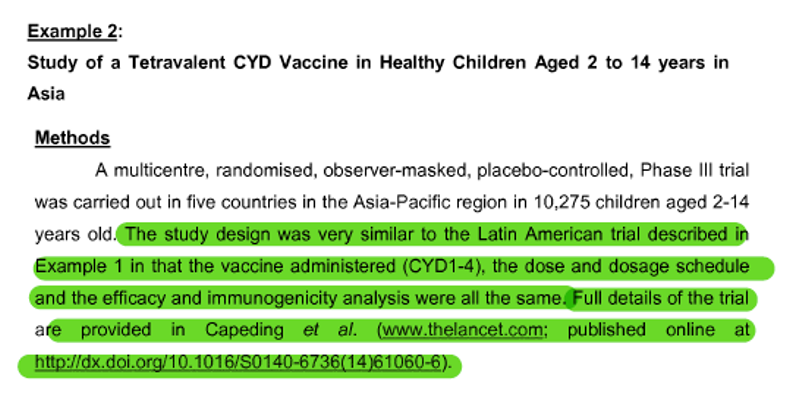
Example 2
(Click image to amplify)
The vaccine composition prepared and administered in Example 2 is identical to that which was administered in Example 1 – using a CYD 1-4 vaccine which relies on the ChimeriVax concept.

Example 3
(Click image to amplify)
This Example only provides an amalgamation of data collected from the clinical trials described in Examples 1 and 2. No change in the vaccine composition has been demonstrated to justify the broad scope of the claims.

Example 4
(Click image to amplify)
Much in the same vein as Example 3, the information disclosed in Example 4 merely refers to a Long-Term Follow-Up (LTFU) of the clinical trials descibed in Examples 1 and 2. Hence, once again there is no justification provided as for the wide scope of the claims.

(1) Exemplo – Sanofi 2

(2) Exemplo – Sanofi 2

(3) Exemplo – Sanofi 2
What is claimed yet not disclosed in the specification?
The claim allows for the inclusion of all types of viruses
While the specification only discloses the ChimeriVax concept – a chimeric virus formed using a Yellow Fever backbone (YF17D) – the claims encompass several types of viruses which could be used in a vaccine for eventually eliciting an immune response against dengue (even those not yet produced or tested). This wide array of claimed subject matter is simply not supported in any way by the scope of the disclosure.
Sanofi 2 – Lack of Clarity and Support of the Claims
-
Industrial Property Statute (LPI)
Article 25 – The claims must be based on the specification, characterizing the particularities of the application and defining clearly and precisely the subject matter to be protected.
-
BRPTO’s Resolution no. 124/2013
2.13 The descriptive sufficiency must be assessed based on the specification, which must disclose the invention in a sufficiently clear and precise manner so as to be reproduced by a person skilled in the art. The specification must contain enough conditions that ensure the achievement of the claimed invention.
2.15. In this context, it must be ensured that the application contains sufficient technical information to enable a person skilled in the art to:
(a) put the invention as claimed into practice, without undue experimentation, and
(b) understand the contribution of the invention to the state of the art to which it belongs.
By undue experimentation it is understood when a person skilled in the art, from the disclosed in the invention, requires additional experimentation to carry it out.3.85. Article 25 of the IPL sets forth that the claims must be based on the specification, characterizing the particularities of the application and clearly and precisely defining the subject matter to be protected. This means that there must be a basis in the specification for the subject matter of every claim and the scope of the claims must not be broader than the content of the specification and drawings, if any, and based on the contribution to the state of the art.
3.86. The proper formulation of a claim must meet the requirement of precision established in Article 25 of the IPL. Most claims are generalizations from one or more particular examples. The extent of generalization permissible is a matter that the examiner must analyze in each particular case in the light of the relevant state of the art.
-
BRPTO’s Resolution no. 169/2016
7.7 Qualitative and/or quantitative definitions, with greater of lesser degree of precision, will only need to be present when they are indispensable to clarity and precision of the claim.
Compositions exclusively defined by the use, form of administration or mechanism of action thereof7.8 Claims of this type are not precise, causing a lack of definition as to the protected subject matter, and should be rejected as set forth in the provisions of article 25 of the IP Statute.
BRPTO’s Resolution no. 208/2017 – Guidelines for Patent Applications in the Field of Chemistry
Composition claims related to a therapeutic regimen cannot be accepted as they lack clarity
6. COMPOSITIONS, FORMULATIONS AND PHYSICAL FORMS OF COMPOSITIONS
6.1. CLARITY AND PRECISENESS OF CLAIMS
Example 11:
Claim 1: Pharmaceutical composition, characterized by comprising compound A and excipients B and C.
Claim 2: Pharmaceutical composition according to claim 1, characterized by the fact that the dosage of A varies from 45 to 90 mg per Kg of the patient.
Not acceptable for lacking clarity (Article 25 of the IPL) since the additional feature of the dependent claim refers to the method of administration of the pharmaceutical composition, which is part of the therapeutic regimen, and is not related to the product. The added characteristic does not add information regarding the product per se, thereby generating inconsistency as to the claimed subject matter.
Claim 3: Pharmaceutical composition according to claim 1, characterized by the fact of being administered 2 times a day.
Not acceptable for lacking clarity (Article 25 of the IPL), since the additional feature of the dependent claim refers to the method of administration of the pharmaceutical composition, which is part of the therapeutic regimen, and is not part of the product. The added characteristic does not add information regarding the product per se, thereby generating inconsistency as to the claimed subject matter.
“Use” claims with features related to a therapeutic regimen cannot be accepted for lack of clarity
9. NOVEL USES OF KNOWN COMPOUNDS
9.1.4. Groundings, clarity and preciseness of the claims
The claims of new use for preparing a medicament should specify the disease to be treated. […]
Fragments related to the therapeutic regimen contained within the new medical use claims and the group of patients neither define the use of the compound for preparing a medicament, and as such, they cannot be accepted for causing uncertainty as to the subject matter.
In this sense, it is clear that the claims of Sanofi 2 lack clarity, whereas:
Claim 1 is a composition claim linked to a therapeutic regimen
and, thus, is not patentable according to art. 25 of the LPI and item 6.1 of the BRPTO’s Resolution no. 208/2017
Claim 15 is an “use” claim linked to a therapeutic regimen
and, thus, is not patentable according to art. 25 of the LPI and item 9.1.4 of the BRPTO’s Resolution no. 208/2017
Claim 16 is a kit comprising the matter of Claim 1
and, thus, is not patentable according to art. 25 of the LPI and item 6.1 of the BRPTO’s Resolution no. 208/2017
Sanofi 2 – Patentability
Relevant Prior Art Documents
D1 – WO2014016362
Date of Publishing: 30/01/2014
Published as: WIPO International Patent Application
Main Disclosures:
- The disclosed invention relates to a dengue virus serotype 2 vaccine composition.
- The patent application describes the results of a Phase IIb clinical Trial conducted by the Applicant, as disclosed in the application as Example 1.
- Strains are selected to compose the prME component of the vaccine against the Dengue caused by serotype 2 Dengue virus based on a Global Consensus Sequence obtained in Example 2: LAV-2 (PDK53-16681); BID-V585; PR/DB023; and MD-1280.
- Tetravalent vaccines are also foreseen in the application.
- Tests with a tetravalent vaccine Chimerivax (CYD-TDV) compared to other Test Tetravalent Formulations in which component of Serotype 2 is varied, are reported.
- Examples 4 and 5 describe comparative tests in Cynomolgus monkeys and naïve adults in Mexico to assess the immunogenicity against DEN-2 for the tested formulations compared to the Chimerivax (CYD-TDV), respectively.
D2 – WO2014016360
Date of Publishing: 30/01/2014
Published as: WIPO International Patent Application
Main Disclosures:
- The disclosed invention relates to a tetravalent vaccine composition against Dengue.
- This a patent application describes the results of a Phase IIb clinical Trial conducted by the Applicant, as disclosed in the application as Example 1.
- Due to an alleged lack of efficacy of the Chimerivax (CYD-TDV) vaccine against DEN-2, like in the WO2014/016362 (D1), a Global Consensus Sequence was developed, and DEN-2 Dengue virus strains have been selected (Example 2), namely: BID-V585; MD-1280; LAV-2 (PDK53-16681); and PR/DB023
- In Example 3, construction of the cDNA clones corresponding to the optimized serotype 2 chimeric viruses selected in Example 2 is described. The virus used as backbone for the constructions is the Yellow Fever strain YF17D204, using Chimerivax technology. No tests with these chimeric strains are reported in this document.
- Example 4 corresponds to the immunogenicity test of CYD 1, 2 and 4-VDV2 composition in Naïve adults in Mexico. This Example is identical to Example 5 from WO2014016362 (D1).
D3 – Bhamarapravati & Sutee
Date of Publishing: 28/03/2000; Date of Publishing (online): 01/09/2010
Published as: Scientific article in the “Vaccine” journal, v. 18, p. 44-47.
Main Disclosures:
- Bhamarapravati is a short communication reporting the developments of dengue vaccines, at the Mahidol University group based on live attenuated (non-chimeric) dengue viruses obtained by serial passages of the viruses in primary dog kidney cells or primary African green monkey kidney cells.
- According to the authors, tetravalent vaccines described have been tested in Thai volunteers, and were found to be immunogenic in both adults and children.
- This document is relevant with regard to the broad component “live attenuated dengue virus” from currently pending claim 1.
D4 – Guy et al.
Date of Publishing: 01/09/2010
Published as: Scientific Article in the “Human Vaccines” journal, v. 6, issue 9, p. 696-705.
Main Disclosures:
- The review is concerned about developments made in relation to Sanofi Pasteur tetravalent vaccine candidate composed of four recombinant live attenuated vaccines based on a yellow fever vaccine 17D (YFV 17D) backbone, each expressing the prM and envelope genes of one of the four dengue virus serotypes, also known as Chimerivax.
- The paper discusses about the overall development of the CYD 1-4 viruses used in the relevant vaccine, i.e., the chimeric yellow fever/dengue (CYD) constructs (see Figure 1); and comments about the Pre-clinical evaluation and Clinical Development of the vaccine, at the occasion. The vaccine composition in this document is the same being tested in application BR112017004197-9 .
- Phase I studies with monovalent CYD-2 and Tetravalent (TDV) vaccine have been discussed in this Review, with seroconversion of volunteers being shown, inclusive for all four serotypes with CYD-TDV (Chimerivax).
- Phase IIb and Phase III clinical evaluations being conducted are mentioned in the paper, thus showing that efficacy tests were being carried out with the vaccine, at the occasion. The phase III trial in children and adolescents in Latin America (Example 1) and Asia (Example 2) are mentioned herein. The phase IIb study from Sanofi 1, and included in the Example 4 (LTFU) of the instant application are also reported.
- The document, therefore, discloses the immunogenicity achieved with the TDV vaccine, the use of the vaccine in humans (children from endemic areas), and the dosage regimen of 0-6-12 months.
D5 – Osorio et al.
Date of Publishing: 21/07/2011
Published as: Scientific Article in the “Vaccine” journal, v. 29, issue 42, p. 7251-7260.
Main Disclosures:
- This document consists of a review article disclosing the results of a chimeric dengue-2 PDK-53-based tetravalent vaccine successfully protecting monkeys in challenge studies.
- The paper describes the attenuated DEN-2 PDK-53 strain and chimeric viruses containing the prM and E genes from DEN-1, DEN-3 and DEN-4, in a DEN-2 PDK-53 backbone, known as DENVax.
- The tetravalent vaccine candidate showed a safety profile and immunogenicity against the four Dengue serotypes, as shown in Phase I Clinical Trials.
- This document is relevant with respect to the broad component “live attenuated chimeric dengue virus” from currently pending claim 1.
Sanofi 2 – Patentability
Lack of Novelty and an Inventive Step
-
Industrial Property Statute (LPI)
Article 8 – To be patentable an invention must meet the requirements of novelty, inventive activity and industrial application
-
Industrial Property Statute (LPI)
Article 11 – Inventions and utility models are considered to be new when not included in the state of the art.
-
Industrial Property Statute (LPI)
Article 13 – An invention shall be taken to involve inventive activity when, for a person skilled in the art, it does not derive in an evident or obvious manner from the state of the art.
-
BRPTO’s Resolution no. 169/2016
7.5 – The compositions not comprised in the prior art are deemed novel. The composition containing component(s) that are already known in the prior art will be deemed novel if it has features, such as, other components and ratio between components, to distinguish it from the state of the art.
-
BRPTO’s Resolution no. 169/2016
7.6 – The effect, use or the mode of administration/release per se do not confer novelty to a composition that is known in the art. However, these elements can be accepted in the text of the claims to provide clarity and precision to the claimed subject matter.
Example: A “pharmaceutical composition characterized by containing X and Y” lacks novelty over a prior art document referring to a detergent composition characterized by containing X and Y.
-
BRPTO’s Resolution no. 208/2017
9.1. NEW MEDICAL USE
9.1.1 Novelty
a disease which is different from that for which the product is employed In order to be considered novel, the new medical use invention must disclose the application of a pharmaceutical product already known in the art for producing a medicament for treating or preventing in the state of the art.
Features related to the use of the compound, such as therapeutic regimen (dosage, administration mode/ application, dosage interval) and/ or group of patients do not render the use of a known compound novel. For example, if the state of the art discloses the “use of compound X for manufacturing a medicament for treating disease Y” and the application seeks protection for the “use of compound X for manufacturing a medicament for treating disease Y for treating diabetic patients”, the claimed use is not considered novel.
Sanofi 1 lacks novelty when compared to D1

The standard Lorem Ipsum passage, used since the 1500s
Contrary to popular belief, Lorem Ipsum is not simply random text. It has roots in a piece of classical Latin literature from 45 BC, making it over 2000 years old. Richard McClintock, a Latin professor at Hampden-Sydney College in Virginia, looked up one of the more obscure Latin words, consectetur, from a Lorem Ipsum passage, and going through the cites of the word in classical literature, discovered the undoubtable source. Lorem Ipsum comes from sections 1.10.32 and 1.10.33 of “de Finibus Bonorum et Malorum” (The Extremes of Good and Evil) by Cicero, written in 45 BC. This book is a treatise on the theory of ethics, very popular during the Renaissance. The first line of Lorem Ipsum, “Lorem ipsum dolor sit amet..”, comes from a line in section 1.10.32.
Sanofi 1 lacks novelty when compared to D2

The standard Lorem Ipsum passage, used since the 1500s
Contrary to popular belief, Lorem Ipsum is not simply random text. It has roots in a piece of classical Latin literature from 45 BC, making it over 2000 years old. Richard McClintock, a Latin professor at Hampden-Sydney College in Virginia, looked up one of the more obscure Latin words, consectetur, from a Lorem Ipsum passage, and going through the cites of the word in classical literature, discovered the undoubtable source. Lorem Ipsum comes from sections 1.10.32 and 1.10.33 of “de Finibus Bonorum et Malorum” (The Extremes of Good and Evil) by Cicero, written in 45 BC. This book is a treatise on the theory of ethics, very popular during the Renaissance. The first line of Lorem Ipsum, “Lorem ipsum dolor sit amet..”, comes from a line in section 1.10.32.
Sanofi 2 – Opinions from Experts in the Field
Renowned experts from the field of immunology attest and reinforce that the Sanofi 2 application DOES NOT meet the requirements for patentability as set forth in the Industrial Property Statute (Public Law no. 9.279/96) and the BRPTO’s Guidelines for Patent Applications (specifically Resolutions no. 169/2016 and 208/2017).
Aliquam dignissim lacinia tristique nulla lobortis nunc ac eros scelerisque varius suspendisse sit amet urna vitae urna semper quis at ligula.
 Dr.ª Alice RayolFarmacêutica, Imunóloga e Doutora em Propriedade Intelectual
Dr.ª Alice RayolFarmacêutica, Imunóloga e Doutora em Propriedade Intelectual
Aliquam dignissim lacinia tristique nulla lobortis nunc ac eros scelerisque varius suspendisse sit amet urna vitae urna semper quis at ligula.
 Dr.ª Ana Claudia Dias de OliveiraBióloga e Doutora em Biotecnologia e Propriedade Intelectual
Dr.ª Ana Claudia Dias de OliveiraBióloga e Doutora em Biotecnologia e Propriedade Intelectual
Sanofi 2 – Takeaways
Due to its lack of conformity with the legal standards for patentability, the Sanofi 2 application should be rejected by the National Institute of Industrial Property (INPI – BRPTO).
Unpatentability of Therapeutic Methods
Art. 10, VIII, of the LPI
Insufficiency of Disclosure
Art. 24 of the LPI
Lack of Clarity and Support of the Claims
Art. 25 of the LPI
Lack of Novelty
Art. 8º and 13 of the LPI
Lack of an Inventive Step
Art. 8º and 13 of the LPI

