Third Party Opposition against Sanofi's Patent Application no. BR 11 2017 028212-7 (YF)
Sanofi seeks to perpetuate an undue protection in the field of vaccination against Dengue.

Yellow Fever (Patent Application no. BR 11 2017 028212-7)
Patent Application no. BR 11 2017 028212-7 does not merit being granted, as it is contrary to many of the legal requirements set forth on Public Law no. 9.729/96 (Industrial Property Statute - LPI)
Unpatentability of Therapeutic Methods
Art. 10, VIII, of the LPI

Insufficiency of Disclosure
Art. 24 of the LPI

Lack of Clarity and Support of the Claims
Art. 25 of the LPI

Lack of Novelty
Art. 8º and 11 of the LPI

Lack of an Inventive Step
Art. 8º and 13 of the LPI
Yellow Fever - Unpatentability of Therapeutic Methods
The current claims of the Yellow Fever application clearly attempt to protect therapeutic methods - which is in explicit violation of the Industrial Property Statute
Industrial Property Statute (LPI)
Article 10 - The following are not considered to be inventions or utility models: […] VIII - operating or surgical techniques and therapeutic or diagnostic methods, for use on the human or animal body; […]
BRPTO's Resolution no. 169/2016
1.27 - Therapeutic methods are those that imply the cure and/or the prevention of a disease or disorder of the human or animal body, or the relief of symptoms of pain, suffering and discomfort, for the purpose of reestablishing or maintaining the normal health conditions thereof.
Text of Hypothetical Claim 1
"A yellow fever (YF) vaccine for use in a method for inducing a protective immune response against yellow fever, characterized in that said method comprises concomitantly administering said yellow fever vaccine to a human subject together with a tetravalent dengue vaccine which comprises a live attenuated dengue virus of each of serotypes 1 to 4,
wherein said method also induces neutralizing antibodies against each of the four serotypes of dengue,
wherein the dengue antigen of serotypes 1, 3 and 4 are each a live attenuated chimeric dengue virus and the dengue antigen of serotype 2 is selected from the group consisting of a live attenuated dengue virus and a live attenuated chimeric dengue virus,
wherein said live attenuated chimeric dengue viruses are recipient flaviviruses in which the genetic backbone has been modified by exchanging the nucleic acid sequences encoding both the pre-membrane (prM) and E proteins of the recipient flavivirus by the corresponding sequences of a dengue virus,
wherein the yellow fever vaccine comprises a live attenuated yellow fever virus strain, and
wherein the human subject is dengue immune, i.e. is a subject who has been previously infected by a dengue virus, and is aged between 12 months and 60 years old"Text of Hypothetical Claim 8
"A tetravalent dengue vaccine which comprises a live attenuated dengue antigen of each of serotypes 1 to 4, for use in a method for inducing a neutralizing antibody response against each of the four serotypes of dengue, characterized in that said method comprises concomitantly administering said tetravalent dengue vaccine to a human subject together with a yellow fever (YF) vaccine, wherein said method also induces protective immune response against yellow fever,
wherein the dengue antigen of serotypes 1, 3 and 4 are each a live attenuated chimeric dengue virus and the dengue antigen of serotype 2 is selected from the group consisting of a live attenuated dengue virus and a live attenuated chimeric dengue virus,
wherein said live attenuated chimeric dengue viruses are recipient flaviviruses in which the genetic backbone has been modified by exchanging the nucleic acid sequences encoding both the pre-membrane (prM) and E proteins of the recipient flavivirus by the corresponding sequences of a dengue virus,
wherein the yellow fever vaccine comprises a live attenuated yellow fever virus strain, and
wherein the human subject is dengue immune, i.e. is a subject who has been previously infected by a dengue virus, and is aged between 12 months and 60 years old."
Yellow Fever - Insufficiency of Disclosure
The current claims of the Yellow Fever application cover subject matter which is not supported by the specification. Thus, the Person of Ordinary Skill in the Art (POSITA) has no means of reproducing the technology without incurring in undue experimentation.
Industrial Property Statute (LPI)
Article 24 - The specification must describe the subject matter clearly and sufficiently so as to enable a person skilled in the art to carry it out and to indicate, when applicable, the best mode of execution.
BRPTO's Resolution no. 124/2013
2.13 The descriptive sufficiency must be assessed based on the specification, which must disclose the invention in a sufficiently clear and precise manner so as to be reproduced by a person skilled in the art. The specification must contain enough conditions that ensure the achievement of the claimed invention.
2.15. In this context, it must be ensured that the application contains sufficient technical information to enable a person skilled in the art to:
(a) put the invention as claimed into practice, without undue experimentation, and
(b) understand the contribution of the invention to the state of the art to which it belongs. By undue experimentation it is understood when a person skilled in the art, from the disclosed in the invention, requires additional experimentation to carry it out.
What is actually disclosed in the specification?

Example 1
(Click image to amplify)
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Ut elit tellus, luctus nec ullamcorper mattis, pulvinar dapibus leo.

Example 2
(Click image to amplify)
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Ut elit tellus, luctus nec ullamcorper mattis, pulvinar dapibus leo.
What is claimed yet not disclosed in the specification?
The claim allows for the inclusion of a large amount of undisclosed viruses
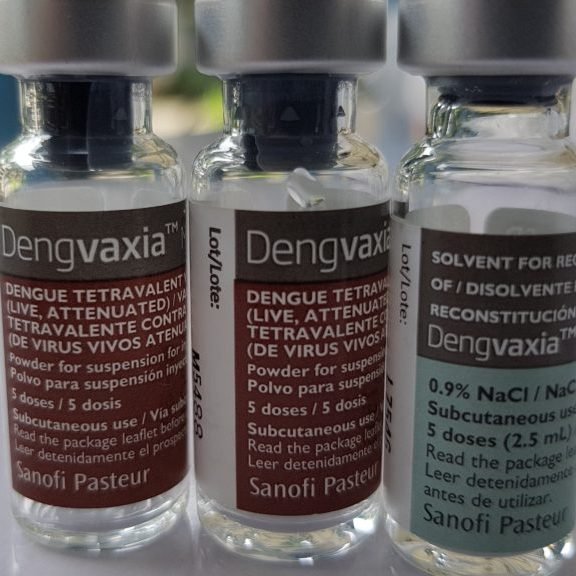
In view of the examples provided in the original description of the specification, there is only support for a vaccine composition consisting of live attenuated chimeric virus, with prM-E nucleotide sequences from dengue virus strains: DEN 1 PU0359 (TYP1 140), DEN2 PU0218, DEN3 PaH881/88 and DEN 4 1228 (TVP 980) in the genomic backbone of Yellow Fever virus strain YF17D. This is the CYD-TDV (Dengvaxia) from Sanofi. For any other vaccine compositions or chimeric virus, no results have been disclosed by the Applicant in the original application to demonstrate the effect of any vaccine or chimeric virus in “inducing a protective immune response against yellow fever” nor in “a neutralizing antibody response against each of the four dengue serotypes” as broadly claimed in the valid set of claims.
Yellow Fever - Lack of Clarity and Support of the Claims
Industrial Property Statute (LPI)
Article 25 - The claims must be based on the specification, characterizing the particularities of the application and defining clearly and precisely the subject matter to be protected.
BRPTO's Resolution no. 124/2013
3.85. Article 25 of the IPL sets forth that the claims must be based on the specification, characterizing the particularities of the application and clearly and precisely defining the subject matter to be protected. This means that there must be a basis in the specification for the subject matter of every claim and the scope of the claims must not be broader than the content of the specification and drawings, if any, and based on the contribution to the state of the art.
3.86. The proper formulation of a claim must meet the requirement of precision established in Article 25 of the IPL. Most claims are generalizations from one or more particular examples. The extent of generalization permissible is a matter that the examiner must analyze in each particular case in the light of the relevant state of the art.BRPTO's Resolution no. 169/2016
7.7 Qualitative and/or quantitative definitions, with greater of lesser degree of precision, will only need to be present when they are indispensable to clarity and precision of the claim. Compositions exclusively defined by the use, form of administration or mechanism of action thereof
7.8 Claims of this type are not precise, causing a lack of definition as to the protected subject matter, and should be rejected as set forth in the provisions of article 25 of the IP Statute.
BRPTO's Resolution no. 208/2017 - Guidelines for Patent Applications in the Field of Chemistry
6. COMPOSITIONS, FORMULATIONS AND PHYSICAL FORMS OF COMPOSITIONS
6.1. CLARITY AND PRECISENESS OF CLAIMS
Example 11:
Claim 1: Pharmaceutical composition, characterized by comprising compound A and excipients B and C.
Claim 2: Pharmaceutical composition according to claim 1, characterized by the fact that the dosage of A varies from 45 to 90 mg per Kg of the patient.
Not acceptable for lacking clarity (Article 25 of the IPL) since the additional feature of the dependent claim refers to the method of administration of the pharmaceutical composition, which is part of the therapeutic regimen, and is not related to the product. The added characteristic does not add information regarding the product per se, thereby generating inconsistency as to the claimed subject matter.
Claim 3: Pharmaceutical composition according to claim 1, characterized by the fact of being administered 2 times a day.
Not acceptable for lacking clarity (Article 25 of the IPL), since the additional feature of the dependent claim refers to the method of administration of the pharmaceutical composition, which is part of the therapeutic regimen, and is not part of the product. The added characteristic does not add information regarding the product per se, thereby generating inconsistency as to the claimed subject matter.
In this sense, it is clear that the claims of Yellow Fever lack clarity, whereas:
Claim 1 features elements which are related to the method of administration
Such features are explicitly deemed to lack clarity according to item 6.1. of the BRPTO's Resolution no. 208/2017. Thus, this claim is not admissable according to art. 25 of the LPI
Claim 1 fails to specify which dengue chimeric viruses it refers to
Such failure to specify the chimeras which are referred in the claim denote a lack of clarity which is not admissible according to art. 25 of the LPI
Claim 1 is a composition claim and features traits related to a group of patients
Such traits extrapolate the composition's own technical features and, thus, lacks clarity and is deemed not patentable according to art. 25 of the LPI
Claim 8 features elements which are related to the method of administration
Such traits extrapolate the composition's own technical features and, thus, lacks clarity and is deemed not patentable according to art. 25 of the LPI
Claim 8 fails to specify which dengue chimeric viruses it refers to
Such failure to specify the chimeras which are referred in the claim denote a lack of clarity which is not admissible according to art. 25 of the LPI
Claim 8 is a composition claim and features traits related to a group of patients
Such traits extrapolate the composition's own technical features and, thus, lacks clarity and is deemed not patentable according to art. 25 of the LPI
Yellow Fever - Patentability
Relevant Prior Art Documents
Yellow Fever - Patentability
Lack of Novelty and an Inventive Step
Industrial Property Statute (LPI)
Article 8 - To be patentable an invention must meet the requirements of novelty, inventive activity and industrial application
Article 11 - Inventions and utility models are considered to be new when not included in the state of the art.
Article 13 - An invention shall be taken to involve inventive activity when, for a person skilled in the art, it does not derive in an evident or obvious manner from the state of the art.
BRPTO's Resolution no. 169/2016
7.5 - The compositions not comprised in the prior art are deemed novel. The composition containing component(s) that are already known in the prior art will be deemed novel if it has features, such as, other components and ratio between components, to distinguish it from the state of the art.
7.6 - The effect, use or the mode of administration/release per se do not confer novelty to a composition that is known in the art. However, these elements can be accepted in the text of the claims to provide clarity and precision to the claimed subject matter.
Example: A "pharmaceutical composition characterized by containing X and Y" lacks novelty over a prior art document referring to a detergent composition characterized by containing X and Y.

The standard Lorem Ipsum passage, used since the 1500s
Contrary to popular belief, Lorem Ipsum is not simply random text. It has roots in a piece of classical Latin literature from 45 BC, making it over 2000 years old. Richard McClintock, a Latin professor at Hampden-Sydney College in Virginia, looked up one of the more obscure Latin words, consectetur, from a Lorem Ipsum passage, and going through the cites of the word in classical literature, discovered the undoubtable source. Lorem Ipsum comes from sections 1.10.32 and 1.10.33 of “de Finibus Bonorum et Malorum” (The Extremes of Good and Evil) by Cicero, written in 45 BC. This book is a treatise on the theory of ethics, very popular during the Renaissance. The first line of Lorem Ipsum, “Lorem ipsum dolor sit amet..”, comes from a line in section 1.10.32.

The standard Lorem Ipsum passage, used since the 1500s
Contrary to popular belief, Lorem Ipsum is not simply random text. It has roots in a piece of classical Latin literature from 45 BC, making it over 2000 years old. Richard McClintock, a Latin professor at Hampden-Sydney College in Virginia, looked up one of the more obscure Latin words, consectetur, from a Lorem Ipsum passage, and going through the cites of the word in classical literature, discovered the undoubtable source. Lorem Ipsum comes from sections 1.10.32 and 1.10.33 of “de Finibus Bonorum et Malorum” (The Extremes of Good and Evil) by Cicero, written in 45 BC. This book is a treatise on the theory of ethics, very popular during the Renaissance. The first line of Lorem Ipsum, “Lorem ipsum dolor sit amet..”, comes from a line in section 1.10.32.
Yellow Fever - Opinions from Experts in the Field
Renowned experts from the field of immunology attest and reinforce that the Yellow Fever application DOES NOT meet the requirements for patentability as set forth in the Industrial Property Statute (Public Law no. 9.279/96) and the BRPTO's Guidelines for Patent Applications (specifically Resolutions no. 169/2016 and 208/2017).


Yellow Fever - Takeaways
Due to its lack of conformity with the legal standards for patentability, the Yellow Fever application should be rejected by the National Institute of Industrial Property (INPI - BRPTO).
Unpatentability of Therapeutic Methods
Art. 10, VIII, of the LPI
Insufficiency of Disclosure
Art. 24 of the LPI
Lack of Clarity and Support of the Claims
Art. 25 of the LPI
Lack of Novelty
Art. 8º and 13 of the LPI
Lack of an Inventive Step
Art. 8º and 13 of the LPI
Scenario 2
Yellow Fever (Patent Application no. BR 11 2017 028212-7)
Patent Application no. BR 11 2017 028212-7 does not merit being granted, as it is contrary to many of the legal requirements set forth on Public Law no. 9.729/96 (Industrial Property Statute - LPI)

Limitation of Voluntary Amendments
Art. 32 of the LPI

Unpatentability of Therapeutic Methods
Art. 10, VIII, of the LPI

Insufficiency of Disclosure
Art. 24 of the LPI

Lack of Clarity and Support of the Claims
Art. 25 of the LPI

Lack of Novelty
Art. 8º and 11 of the LPI

Lack of an Inventive Step
Art. 8º and 13 of the LPI
Yellow Fever - Limitations on Voluntary Amendments
The current claims of the Yellow Fever application clearly extrapolate the limits which Brazilian statute imposes on the possibility of requesting amendments post examination request.
Industrial Property Statute (LPI)
Article 32 - In order better to clarify or define a patent application, the applicant may effect alterations up to the request for examination, provided that they be limited to the subject matter initially disclosed in the application.
BRPTO's Resolution no. 93/2013
2.2. Regarding the amendments not to be allowed to the SC
(i) After the request for examination of the patent application, any amendments which purport to broaden the CLAIMED subject matter will not be acceptable.
Text of Original Independent Claim 1
"A yellow fever (YF) vaccine for use in a method for inducing a protective immune response against yellow fever, characterized in that said method comprises concomitantly administering said yellow fever vaccine to a human subject together with a tetravalent dengue vaccine which comprises a live attenuated dengue virus of each of serotypes 1 to 4".
Text of Original Independent Claim 8
"A tetravalent dengue vaccine which comprises a live attenuated dengue antigen of each of serotypes 1 to 4, for use in a method for inducing a protective immune response against yellow fever, characterized in that said method comprises concomitantly administering said tetravalent dengue vaccine to a human subject together with a yellow fever (YF) vaccine".
Text of Pending Independent Claim 1 (April 2021)
"Use of a vaccine against yellow fever (YF) and a tetravalent vaccine against dengue that comprises an attenuated dengue virus of each of serotypes 1 to 4, characterized it is for the manufacture of vaccine compositions for simultaneously inducing a protective immune response against yellow fever and a neutralizing antibody response against each of the four dengue serotypes in a human subject".
Text of Pending Independent Claim 8 (April 2021)
"A tetravalent dengue vaccine which comprises a live attenuated dengue antigen of each of serotypes 1 to 4, for use in a method for inducing a protective immune response against yellow fever, characterized in that said method comprises concomitantly administering said tetravalent dengue vaccine to a human subject together with a yellow fever (YF) vaccine".
Text of Pending Independent Claim 15 (April 2021)
"A vaccine kit characterized in that it comprises a vaccine against YF and a tetravalent vaccine against dengue that comprises a live attenuated dengue virus of each of serotypes 1 to 4".
Text of Pending Independent Claim 16 (April 2021)
"Mixture of a yellow fever vaccine and a dengue tetravalent vaccine, characterized in that it comprises a live attenuated dengue virus of each of serotypes 1 to 4".
Text of Pending Independent Claim 17 (April 2021)
"Use of a live, attenuated vaccine against yellow fever (YF), characterized it is for the manufacture of vaccine compositions for simultaneously inducing a protective immune response against yellow fever and a neutralizing antibody response against each of the four dengue serotypes in a human subject".
Yellow Fever - Unpatentability of Therapeutic Methods
The current claims of the Yellow Fever application clearly attempt to protect therapeutic methods - which is in explicit violation of the Industrial Property Statute
Industrial Property Statute (LPI)
Article 10 - The following are not considered to be inventions or utility models: […] VIII - operating or surgical techniques and therapeutic or diagnostic methods, for use on the human or animal body; […]
BRPTO's Resolution no. 169/2016
1.27 - Therapeutic methods are those that imply the cure and/or the prevention of a disease or disorder of the human or animal body, or the relief of symptoms of pain, suffering and discomfort, for the purpose of reestablishing or maintaining the normal health conditions thereof.
Text of Original (and valid) Claim 1
"A yellow fever (YF) vaccine for use in a method for inducing a protective immune response against yellow fever, characterized in that said method comprises concomitantly administering said yellow fever vaccine to a human subject together with a tetravalent dengue vaccine which comprises a live attenuated dengue virus of each of serotypes 1 to 4".
Text of Original (and valid) Claim 8
"A tetravalent dengue vaccine which comprises a live attenuated dengue antigen of each of serotypes 1 to 4, for use in a method for inducing a protective immune response against yellow fever, characterized in that said method comprises concomitantly administering said tetravalent dengue vaccine to a human subject together with a yellow fever (YF) vaccine"
Yellow Fever - Insufficiency of Disclosure
The current claims of the Yellow Fever application cover subject matter which is not supported by the specification. Thus, the Person of Ordinary Skill in the Art (POSITA) has no means of reproducing the technology without incurring in undue experimentation.
Industrial Property Statute (LPI)
Article 24 - The specification must describe the subject matter clearly and sufficiently so as to enable a person skilled in the art to carry it out and to indicate, when applicable, the best mode of execution.
BRPTO's Resolution no. 124/2013
2.13 The descriptive sufficiency must be assessed based on the specification, which must disclose the invention in a sufficiently clear and precise manner so as to be reproduced by a person skilled in the art. The specification must contain enough conditions that ensure the achievement of the claimed invention.
2.15. In this context, it must be ensured that the application contains sufficient technical information to enable a person skilled in the art to:
(a) put the invention as claimed into practice, without undue experimentation, and
(b) understand the contribution of the invention to the state of the art to which it belongs. By undue experimentation it is understood when a person skilled in the art, from the disclosed in the invention, requires additional experimentation to carry it out.BRPTO's Resolution no. 124/2013
3.85. Article 25 of the IPL sets forth that the claims must be based on the specification, characterizing the particularities of the application and clearly and precisely defining the subject matter to be protected. This means that there must be a basis in the specification for the subject matter of every claim and the scope of the claims must not be broader than the content of the specification and drawings, if any, and based on the contribution to the state of the art.
3.86. The proper formulation of a claim must meet the requirement of precision established in Article 25 of the IPL. Most claims are generalizations from one or more particular examples. The extent of generalization permissible is a matter that the examiner must analyze in each particular case in the light of the relevant state of the art.
What is actually disclosed in the specification?

Example 1
(Click image to amplify)
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Ut elit tellus, luctus nec ullamcorper mattis, pulvinar dapibus leo.

Example 2
(Click image to amplify)
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Ut elit tellus, luctus nec ullamcorper mattis, pulvinar dapibus leo.
What is claimed yet not disclosed in the specification?

The claim allows for a much broader interpretation than what is revealed
In view of the examples provided in the original description of the specification, there is only support for a vaccine composition consisting of live attenuated chimeric virus, with prM-E nucleotide sequences from dengue virus strains: DEN 1 PU0359 (TYP1 140), DEN2 PU0218, DEN3 PaH881/88 and DEN 4 1228 (TVP 980) in the genomic backbone of Yellow Fever virus strain YF17D. This is the CYD-TDV (Dengvaxia) from Sanofi. For any other vaccine compositions or chimeric virus, no results have been disclosed by the Applicant in the original application to demonstrate the effect of any vaccine or chimeric virus in “inducing a protective immune response against yellow fever” nor in “a neutralizing antibody response against each of the four dengue serotypes” as broadly claimed in the valid set of claims.
Yellow Fever - Lack of Clarity and Support of the Claims
Industrial Property Statute (LPI)
Article 25 - The claims must be based on the specification, characterizing the particularities of the application and defining clearly and precisely the subject matter to be protected.
BRPTO's Resolution no. 124/2013
3.85. Article 25 of the IPL sets forth that the claims must be based on the specification, characterizing the particularities of the application and clearly and precisely defining the subject matter to be protected. This means that there must be a basis in the specification for the subject matter of every claim and the scope of the claims must not be broader than the content of the specification and drawings, if any, and based on the contribution to the state of the art.
3.86. The proper formulation of a claim must meet the requirement of precision established in Article 25 of the IPL. Most claims are generalizations from one or more particular examples. The extent of generalization permissible is a matter that the examiner must analyze in each particular case in the light of the relevant state of the art.BRPTO's Resolution no. 169/2016
7.7 Qualitative and/or quantitative definitions, with greater of lesser degree of precision, will only need to be present when they are indispensable to clarity and precision of the claim. Compositions exclusively defined by the use, form of administration or mechanism of action thereof
7.8 Claims of this type are not precise, causing a lack of definition as to the protected subject matter, and should be rejected as set forth in the provisions of article 25 of the IP Statute.
BRPTO's Resolution no. 208/2017 - Guidelines for Patent Applications in the Field of Chemistry
6. COMPOSITIONS, FORMULATIONS AND PHYSICAL FORMS OF COMPOSITIONS
6.1. CLARITY AND PRECISENESS OF CLAIMS
Example 11:
Claim 1: Pharmaceutical composition, characterized by comprising compound A and excipients B and C.
Claim 2: Pharmaceutical composition according to claim 1, characterized by the fact that the dosage of A varies from 45 to 90 mg per Kg of the patient.
Not acceptable for lacking clarity (Article 25 of the IPL) since the additional feature of the dependent claim refers to the method of administration of the pharmaceutical composition, which is part of the therapeutic regimen, and is not related to the product. The added characteristic does not add information regarding the product per se, thereby generating inconsistency as to the claimed subject matter.
Claim 3: Pharmaceutical composition according to claim 1, characterized by the fact of being administered 2 times a day.
Not acceptable for lacking clarity (Article 25 of the IPL), since the additional feature of the dependent claim refers to the method of administration of the pharmaceutical composition, which is part of the therapeutic regimen, and is not part of the product. The added characteristic does not add information regarding the product per se, thereby generating inconsistency as to the claimed subject matter.
In this sense, it is clear that the original (and valid) claims of Yellow Fever lack clarity, whereas:
Claim 1 features elements which are related to a method of treatment and administration
Such features are explicitly deemed to lack clarity according to item 6.1. of the BRPTO's Resolution no. 208/2017. Thus, this claim is not admissable according to art. 25 of the LPI
Claim 8 features elements which are related to the method of treatment and administration
Such features are explicitly deemed to lack clarity according to item 6.1. of the BRPTO's Resolution no. 208/2017. Thus, this claim is not admissable according to art. 25 of the LPI
Yellow Fever - Patentability
Relevant Prior Art Documents
Yellow Fever - Patentability
Lack of Novelty and an Inventive Step
Industrial Property Statute (LPI)
Article 8 - To be patentable an invention must meet the requirements of novelty, inventive activity and industrial application
Article 11 - Inventions and utility models are considered to be new when not included in the state of the art.
Article 13 - An invention shall be taken to involve inventive activity when, for a person skilled in the art, it does not derive in an evident or obvious manner from the state of the art.
BRPTO's Resolution no. 169/2016
7.5 - The compositions not comprised in the prior art are deemed novel. The composition containing component(s) that are already known in the prior art will be deemed novel if it has features, such as, other components and ratio between components, to distinguish it from the state of the art.
7.6 - The effect, use or the mode of administration/release per se do not confer novelty to a composition that is known in the art. However, these elements can be accepted in the text of the claims to provide clarity and precision to the claimed subject matter.
Example: A "pharmaceutical composition characterized by containing X and Y" lacks novelty over a prior art document referring to a detergent composition characterized by containing X and Y.

The standard Lorem Ipsum passage, used since the 1500s
Contrary to popular belief, Lorem Ipsum is not simply random text. It has roots in a piece of classical Latin literature from 45 BC, making it over 2000 years old. Richard McClintock, a Latin professor at Hampden-Sydney College in Virginia, looked up one of the more obscure Latin words, consectetur, from a Lorem Ipsum passage, and going through the cites of the word in classical literature, discovered the undoubtable source. Lorem Ipsum comes from sections 1.10.32 and 1.10.33 of “de Finibus Bonorum et Malorum” (The Extremes of Good and Evil) by Cicero, written in 45 BC. This book is a treatise on the theory of ethics, very popular during the Renaissance. The first line of Lorem Ipsum, “Lorem ipsum dolor sit amet..”, comes from a line in section 1.10.32.

The standard Lorem Ipsum passage, used since the 1500s
Contrary to popular belief, Lorem Ipsum is not simply random text. It has roots in a piece of classical Latin literature from 45 BC, making it over 2000 years old. Richard McClintock, a Latin professor at Hampden-Sydney College in Virginia, looked up one of the more obscure Latin words, consectetur, from a Lorem Ipsum passage, and going through the cites of the word in classical literature, discovered the undoubtable source. Lorem Ipsum comes from sections 1.10.32 and 1.10.33 of “de Finibus Bonorum et Malorum” (The Extremes of Good and Evil) by Cicero, written in 45 BC. This book is a treatise on the theory of ethics, very popular during the Renaissance. The first line of Lorem Ipsum, “Lorem ipsum dolor sit amet..”, comes from a line in section 1.10.32.
Yellow Fever - Opinions from Experts in the Field
Renowned experts from the field of immunology attest and reinforce that the Yellow Fever application DOES NOT meet the requirements for patentability as set forth in the Industrial Property Statute (Public Law no. 9.279/96) and the BRPTO's Guidelines for Patent Applications (specifically Resolutions no. 169/2016 and 208/2017).


Yellow Fever - Takeaways
Due to its lack of conformity with the legal standards for patentability, the Yellow Fever application should be rejected by the National Institute of Industrial Property (INPI - BRPTO).
Unpatentability of Therapeutic Methods
Art. 10, VIII, of the LPI
Insufficiency of Disclosure
Art. 24 of the LPI
Lack of Clarity and Support of the Claims
Art. 25 of the LPI
Lack of Novelty
Art. 8º and 13 of the LPI
Lack of an Inventive Step
Art. 8º and 13 of the LPI

